Molecular spectra entails the absorption of electromagnetic radiation by molecules. This includes vibrational and rotational transitions as well as electronic transitions. The transitions of molecules are more complex than those of atoms. This is because molecules have interactions from vibrations within the molecule associated with the stretching or bending of bonds between atoms, or the rotation of the molecule about its center of gravity. Molecules may have some kinetic energy associated with its straight-line motion in a certain direction. The energy levels involved with these various transitions differ between one another. The energy with the movement of an electron from one orbital to another, electronic, is about 10-9 joules. The energy involved in vibration is about 10-19 joules and that of rotational is around10-21 joules. The energy of transitions change is much smaller than those at about 10-35 joules. Each electronic state of a molecule has several possible vibrational states and each of those has several rotational states. Example follows:
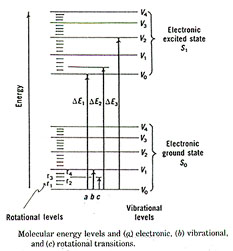
The energy absorbed by a molecule has specific wavelengths in ultraviolet, visible and infrared regions. The study of these specific wavelengths in relation to liquids and solutions is discussed in the section of UV/V spectrophotometry.
The Guide To Acing Physical Pharmacy |